PAA or Peracetic Acid has been somewhat of a buzz word in recent years, not only in the safety and health world but across numerous well-known industries. The use and market share of this chemical has grown rapidly in the last 5 years. This is due to its myriad of applications, it’s ease of use, effectiveness and the fact that it does not leave behind any toxic residues. With its many advantages, it is hard to find another chemical like it.
PAA is instrumental in ensuring that a variety of products are safe for consumer use. It is a chemical that serves as both disinfectant and sanitizer in the healthcare, wastewater treatment, food industries and beyond. Produced by reacting acetic acid and hydrogen peroxide with an acid catalyst, peracetic acid is always sold in stabilized solutions containing acetic acid, hydrogen peroxide, and water. For the food and healthcare industries, peracetic acid is typically sold in concentrates of 1 to 5 percent and is diluted before use.
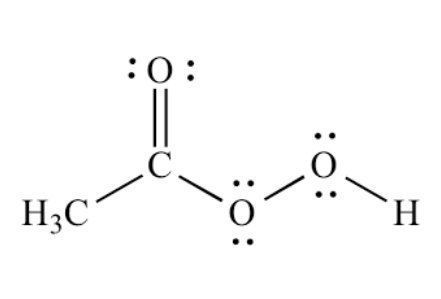
PAA can also be known as peroxyacetic acid, peracetic acid, periacetic acid or per acid. It is a clear, colorless liquid, known for being a strong oxidizing agent. Those electrons you see in the above chemical structure play an important role in making PAA such successful disinfectant. It usually has a strong, vinegar (acetic acid) like odor. PAA degrades rapidly, leaves little to no residue, and decomposes into relatively harmless naturally occurring substances. Its decomposition products are acetic acid, oxygen and water. It is known for being environmentally friendly because unlike other sanitizers, no rinse is required. PAA Can be used as a sanitizer, disinfectant or sterilizer…It is just a matter of contact time and concentration.